Abstract
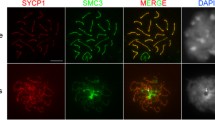
The sensitivity to DNase I of the meiotic sex chromosomes of the male mouse was determined by in situ nick translation. At pachytene and diakinesis-metaphase I, six segments, four at the ends of the X and Y chromosomes and two at internal sites on the X chromosome, were found to be more sensitive than the other parts of these chromosomes. The sensitive segments presumably reflect an active or potentially active chromatin conformation which is maintained in the sex chromosomes despite the earlier reported, almost complete cessation of uridine incorporation. The distribution of regions which are sensitive to DNase I corresponds to that of early DNA replication bands. Active conformation patterns like those figured here, probably exist in the sex chromosomes of other mammals as well.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Chandley AC, McBeath S (1987) Mapping of DNAase I sensitive sites on human meiotic chromosomes by in situ nick translation. Chromosomes Today 9, in press
Fletcher JM (1979) Light microscope analysis of meiotic prophase chromosomes by silver staining. Chromosoma 72:241–248
Gartler SM, Riggs AD (1983) Mammalian X-chromosome inactivation. Annu Rev Genet 17:155–190
Henderson SA (1964) RNA synthesis during male meiosis and spermiogenesis. Chromosoma 15:345–366
Hotta Y, Chandley AC (1982) Activities of X-linked enzymes in spermatocytes of mice rendered sterile by chromosomal alterations. Gam Res 6:65–72
Hotta Y, Chandley AC, Stern H (1977) Biochemical analysis of meiosis in the male mouse. II. DNA metabolism at pachytene. Chromosoma 62:255–268
Hoy RR, Hahn J, Paul RC (1977) Hybrid cricket auditory behavior: Evidence for genetic coupling in animal communication. Science 195:82–84
Jablonka E, Goitein R, Marcus M, Cedar H (1985) DNA hypomethylation causes an increase in DNase-I sensitivity and an advance in the time of replication of the entire inactive X chromosome. Chromosoma 93:152–156
Jablonka E, Goitein R, Sperling K, Cedar H, Marcus M (1987) 5-aza-C-induced changes in the time of replication of the X chromosomes of Microtus agrestis are followed by non-random reversion to a late pattern of replication. Chromosoma 95:81–88
Kerem B, Goitein R, Richler C, Marcus M, Cedar H (1983) In situ nick-translation distinguishes between active and inactive X chromosomes. Nature 304:88–90
Kerem B, Goitein R, Diamond G, Cedar H, Marcus M (1984) Mapping of DNAase I sensitive regions on mitotic chromosomes. Cell 38:493–499
Kierszenbaum AL, Tres LL (1974) Nucleolar and perichromosomal RNA synthesis during meiotic prophase in the mouse testis. J Cell Biol 60:39–53
Lifschytz E, Lindsley DL (1972) The role of X-chromosome inactivation during spermatogenesis. Proc Natl Acad Sci USA 69:182–186
Liskay RM, Evans RJ (1980) Inactive X chromosome DNA does not function in DNA-mediated cell transformation for the hypoxanthine phosphoribosyl transferase gene. Proc Natl Acad Sci USA 77:4895–4898
Lyon MF (1961) Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature 190:372–373
Messthaler H, Traut W (1975) Phases of sex chromosome inactivation in Oncopeltus fasciatus and Pyrrhocoris apterus (Insecta, Heteroptera). Caryologia 28:501–510
Monesi V (1965) Synthetic activities during spermatogenesis in the mouse. RNA and protein. Exp Cell Res 39:197–224
Murer-Orlando ML, Peterson AC (1985) In situ nick translation of human and mouse chromosomes detected with a biotinylated nucleotide. Exp Cell Res 157:322–334
Raman R, Nanda I (1986) Mammalian sex chromosomes. I. Cytological changes in the chiasmatic sex chromosomes of the male musk-shrew Suncus murinus. Chromosoma 93:367–374
Rao SRV, Arora P (1979) Insect sex chromosomes. III. Differential susceptibility of homologous X chromosomes of Gryllotalpa fossor to 3H-Urd-induced aberrations. Chromosoma 74:241–252
Rastan S, Robertson EJ (1985) X-chromosome deletions in embryo-derived (EK) cell lines associated with lack of X-chromosome inactivation. J Embryol Exp Morphol 90:379–388
Rosenmann A, Wahrman J, Richler C, Voss R, Persitz A, Goldman B (1985) Meiotic association between the XY chromosomes and unpaired autosomal elements as a cause of human male sterility. Cytogenet Cell Genet 39:19–29
Somssich I, Hameister H, Winking H (1981) The pattern of early replicating bands in the chromosomes of the mouse. Cytogenet Cell Genet 30:222–231
Sperling K, Marcus M (1984) Mapping of genetic activity on mammalian chromosomes. Chromosomes Today 8:169–178
Stalder J, Groudine M, Dodgson JB, Engel JD, Weintraub H (1980) Hb switching in chickens. Cell 19:973–980
Venolia L, Cooper DW, O'Brien DA, Millette CF, Gartler SM (1984) Transformation of the Hprt gene with DNA from spermatogenic cells. Implications for the evolution of X chromosome inactivation. Chromosoma 90:185–189
Weisbrod S (1982) Active chromatin. Nature 297:289–295
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Richler, C., Uliel, E., Kerem, BS. et al. Regions of active chromatin conformation in ‘inactive’ male meiotic sex chromosomes of the mouse. Chromosoma 95, 167–170 (1987). https://doi.org/10.1007/BF00330346
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00330346